Technology Platform
Realizing the promise of genome editing

Pioneering the Future of Medicine
Genome editing technologies allow genetic material to be removed, added, or altered at a specific location in the genome to develop life-changing therapies for the most challenging genetic disorders.
Our next-generation gene editing platform offers one of the world’s largest and most diverse libraries of RNA-guided nucleases (RGNs), base editors, and reverse transcriptase editors, that provide flexible editing and unprecedented access to the genome.
We have identified a wide range of CRISPR-Cas systems, including known subtypes, and engineered select systems into a variety of editing modalities. Powered by artificial intelligence, we are rapidly progressing CRISPR discovery and engineering efforts to enable the development of gene editing therapies for a broad range of challenging disorders.
Diverse Tools for Unlimited Gene Editing
PAM Diversity is Key to Making Any Edit, Anywhere Possible
A key advantage of our nuclease collections is in the diversity of Protospacer Adjacent Motifs (PAMs), which offer unprecedented flexibility across a full spectrum of gene editing modalities. PAMs are short sequences that determine the DNA segments in the genome to which a nuclease can bind to edit a gene.
Our broad range of PAMs increase the number of specific sites where therapeutically meaningful edits can be made, granting our nucleases access to virtually any region of the genome.
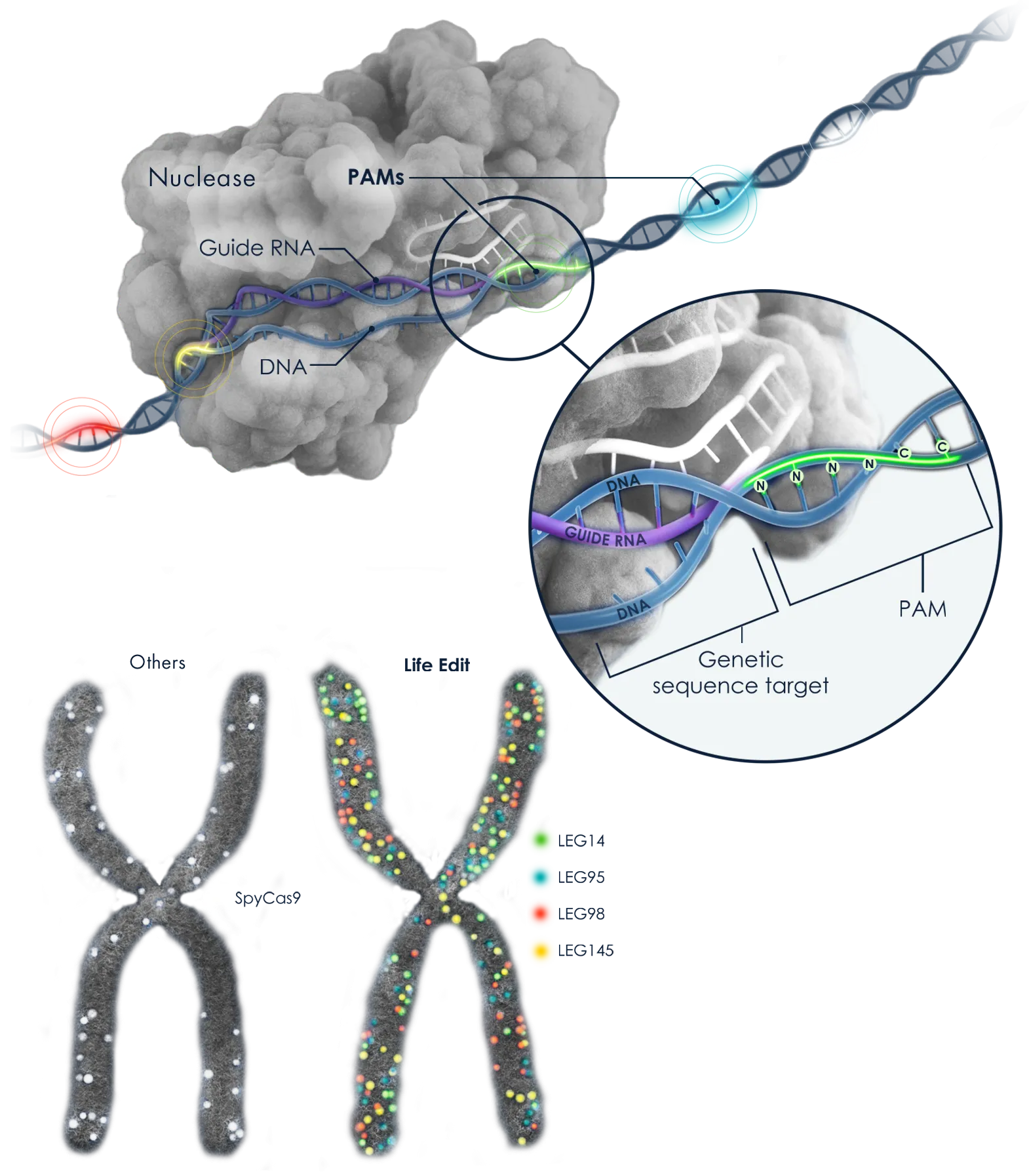
Full Spectrum of Gene Editing Modalities
We have versatile editing options for targeting genetic disorders. The diversity of PAMs in our lead systems offer unprecedented flexibility in three types of gene editing.
Double-Strand Breaks
Double-strand breaks involve the nuclease cutting both strands of the DNA, enabling gene deletion (knock-out) or insertion (knock-in) at the cut site. The PAM diversity of our nucleases allows the introduction of knock-out or knock-in edits virtually anywhere in the genome.
Base Editing
Base editing converts one nucleotide (base) into another, without cutting both strands of DNA. This is achieved by coupling a nuclease, modified to cut only one DNA strand, to a deaminase that edits the target nucleotide. Our modular approach to base editing couples our proprietary nucleases deaminases to one another.
The PAM diversity of our nucleases addresses a critical base editing parameter in which the target mutation must fall within a tighter “window” than required for double-strand breaks. Our PAM diversity enables base editing at more sites than any one nuclease could access.
Our base editing systems includes A and C base editors for ex vivo and in vivo applications with demonstrated multiplex editing capabilities. Base editing technology allows precise nucleotide-level correction of harmful mutations or disruption of coding sequences to reduce gene expression.
Reverse Transcriptase (RT) Editing
RT editing involves cutting one DNA strand, then replacing an existing DNA sequence – from the cut site to the target editing site – with a new sequence that is encoded by the guide RNA (gRNA). Our approach takes advantage of our panel of RGNs – coupled with our expertise in target screening, analysis, and optimization – to enable optimal editing of the target locus.
The PAM diversity of our platform is also able to position the nuclease as close to the editing site as possible, reducing the size of the required gRNA and the challenges associated with lengthy gRNAs. RT editing allows the most precise and flexible editing outcomes for many different types of gene correction, insertion, or knock-out.
Our RNA-Guided Nucleases
Our RNA-guided nucleases were developed using a proprietary collection of non-pathogenic microbes, which offer gene editing tools with higher fidelity, novel functionality, and easier delivery.
Our RGNs are smaller in size (~800-1,100 aa) when compared to conventional nucleases, potentially enabling greater versatility in packaging our systems for therapeutic delivery.
Therapeutic Delivery
We have broad therapeutic delivery capabilities, including viral and non-viral delivery platforms to provide flexibility in therapeutic design and application, unlocking new possibilities for addressing a wide range of diseases and disorders.
Viral Delivery Systems
Our adeno-associated virus (AAV) vectors are a method to deliver DNA to cells or tissues to target disease. The AAV vectors are engineered to transport genetic material into the body, which then instructs cells to provide the desired therapeutic effect.
Non-Viral Delivery Systems
Our novel lipid nanoparticle (LNP) platform has both liver targeting and de-targeting potential, enhancing our ability to effectively deliver gene editing therapies and unlocking new possibilities for addressing a wide range of diseases and disorders. The platform’s adaptability also has the potential to expand its targeting capabilities beyond the liver.
Harnessing Artificial Intelligence to Accelerate CRISPR Discovery and Design
We believe we have one of the largest CRISPR data sets in the industry with a wide range of CRISPR-Cas systems, including known subtypes identified, which engineered select systems into a variety of editing modalities, and with bases of assembled and formatted sequences. We also have an extensive catalog of more than 8 billion proteins for rapid homology searching, enabling our scientists to efficiently identify new targets.
Harnessing the power of artificial intelligence (AI), we propel CRISPR discovery and engineering efforts at an unprecedented pace to enable the development of promising therapeutic options for the broadest range of genetic disorders and diseases that were once deemed untreatable. To achieve this, we believe we have to look beyond nature-derived CRISPR systems to the design of synthetic CRISPRs that can be tailored for each disease. We believe leveraging AI will accelerate the path towards this goal.

Our Therapeutic Approaches
We are enabling the development of a range of ex vivo and in vivo cell and gene editing therapies, leveraging expertise to design and develop an array of modalities.
Partner with Life Edit
We are looking to form strategic partnerships with biotech and pharmaceutical companies to advance their life-changing and curative therapies. Our flexible partnership options include broad R&D collaborations, exclusive and non-exclusive licenses for specific assets, and strategic partnerships to access our technology platforms designed to accelerate the pipelines of our partners.
To provide access to genome editing tools and cure disease, we’re seeking partnerships with biotech and pharmaceutical companies including those interested in ex vivo therapies. We will also continue to support our broad portfolio of innovative biotech companies.
